
An artificial heart is a prosthetic device that is implanted into the body to replace the biological heart. It is distinct from a cardiopulmonary bypass machine (CPB) / Heart-Lung machine, which is an external device used to provide the functions of both the heart and the lungs.


The CPB oxygenates the blood, so does not need to be connected to both blood circuits. Also, a CPB is only suitable for a few hours use, while artificial hearts have so far been used for periods of long over a year.

(ARTIFICIAL HEART)
Origins
A synthetic replacement for the heart remains one of the long-sought holy grails of modern medicine.

(HEART TRANSPLANT)

(HEART TRANSPLANT)
The obvious benefit of a functional artificial heart would be to lower the need for heart transplants, because the demand for donor hearts (as it is for all organs) always greatly exceeds supply.

(ARTIFICIAL HEAT PARTS)
Although the heart is conceptually simple (basically a muscle that functions as a pump), it embodies subtleties that defy straightforward emulation with synthetic materials and power supplies. Consequences of these issues include severe foreign-body rejection and external batteries that limit patient mobility. These complications limited the lifespan of early human recipients to hours or days.
EARLY DESIGNS
A heart-lung machine was used in 1953 during the first successful open heart surgery.

(FIRST HEART-LUNG MACHINE)
Dr. John Heysham Gibbon performed the operation and developed the heart-lung substitute himself. Whether this device could be considered as an artificial heart is a subject of debate.

(Dr.John Gibbon, Mrs.Mary Gibbon & Heart-Lung Machine)
The first patented artificial heart was invented by Paul Winchell in 1963.

Winchell subsequently assigned the patent to the University of Utah, where Robert Jarvik ultimately used it as the model for his Jarvik-7.

Jarvik-7 Artificial Heart, front view (left), back view (right)
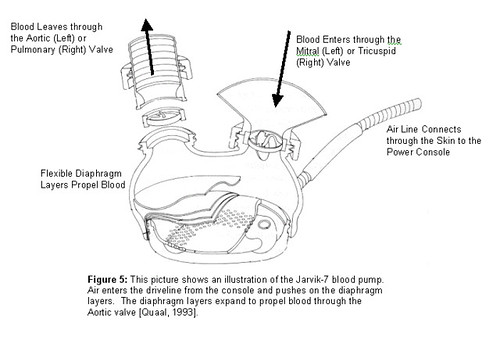
Jarvik's designs improved the device, but his patients succumbed after brief trials. His first Jarvik-7 patient, 61-year-old retired dentist Barney Clark, survived for 112 days after it was implanted at the University of Utah on December 2, 1982.

(Dr. Robert Jarvik, inventor of the artificial heart, Jarvik-7, holds up a model like the one implanted in Barney Clark on Dec. 3, 1982. )
One of the innovations of the Jarvik-7 was the inner coating of rough material, developed by David Gernes. This coating helped the blood to clot and coat the inside of the device, enabling a more natural blood flow.
After about 90 people received the Jarvik device, the implantation of artificial hearts was banned for permanent use in patients with heart failure, because most of the recipients could not live more than half a year. However, it is used temporarily for some heart transplantation candidates who cannot find a natural heart immediately but urgently need an efficiently working heart.
Hiroaki Harasaki of the Cleveland Clinic developed two important improvements for the artificial heart and projected future artificial organs.

(Faculty researchers (from left) Jaikrishnan Kadambi and Hiroaki Harasaki and graduate student Stefan Baumann examine some of the features of their pulsatile heart loop that mechanically simulates how the blood flows through the heart.)
The two patented inventions solved major obstacles for any fully implanted artificial organs and materials. The first was a non-clotting surface material which significantly reduces the risk of rejection of the organ by the patient's immune system. The second development, which required the collaboration of many disciplines, was an implantable power source which does not create tissue-damaging heat.
DIFFERENCE BETWEEN JARVIK & ABIOCOR ARTIFICIAL HEARTS
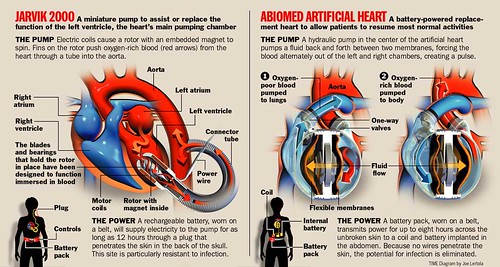
(CLICK PICTURE TO ENLARGE)
Recent developments

On July 2, 2001, Robert Tools received the AbioCor Implantable Replacement Heart produced by the AbioMed company of Danvers, Massachusetts. It was the first completely self-contained artificial heart transplant. The surgery was done by University of Louisville doctors at Jewish Hospital in Louisville, Kentucky.

Tom Christerson survived for 17 months after another AbioCor transplant. On September 6, 2006 the AbioCor device became the first fully implantable artificial heart to be approved under 'Humanitarian Use Device' rules.

The 'CardioWest' temporary Total Artificial Heart (TAH‑t) was developed from the Jarvik-7 by University of Arizona researchers and approved for use in 2004. It is the first implantable artificial heart to be approved by the U.S. Food and Drug Administration, and has also been approved by the CE.

(CARDIOWEST TAH-t ARTIFICIAL HEART)
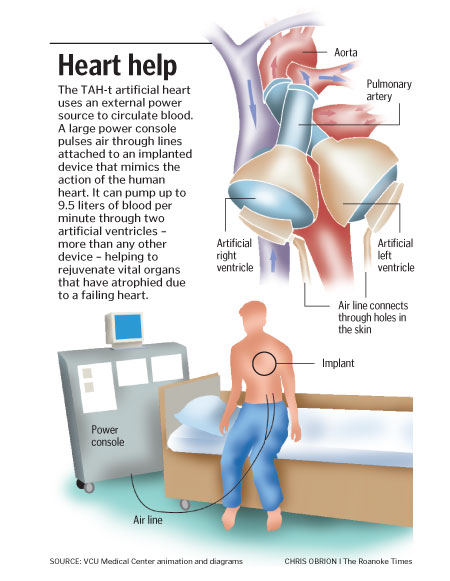
The TAH-t is used only in patients with end stage biventricular failure as a way to improve life expectancy while they are waiting for a heart transplant. In a pivotal clinical study, these patients were successfully transplanted 79% of the time; One-year and five-year survival rates after heart transplant among these patients were 86 and 64 percent. The longest TAH‑t implantation so far went 602 days (20.4 months). There are several medical centers where this device can be implanted:
United States:
- University Medical Center (Tucson, AZ)
- Cleveland Clinic (Cleveland, OH)
- Virginia Commonwealth University Health System (Richmond, VA)
- Aurora St. Luke's (Milwaukee, WI)
- University of Michigan Health System (Ann Arbor, MI)
- Penn State Hershey Medical Center (Hershey, PA)
- Ohio State University Medical Center (Columbus, OH)
- Hospital of the University of Pennsylvania (Philadelphia, PA)
- Barnes Jewish Hospital (St. Louis, MO)
Canada:
- Montreal Heart Institute (Quebec, Canada)
Europe:
- Groupe Hospitalier La Pitié-Salpêtrière (Paris, France)
- Hôpital Guillaume et René Laennec (Nantes, France)
- Deutsches Herzzentrum Berlin / German Heart Institute Berlin (Berlin, Germany)
- Herz-und Diabeteszentrum Nordrhein Westfalen / Heart and Diabetes Center (Bad Oeynhausen, Germany)
- Herzzentrum Leipzig GmbH Universitaetsklinik (Leipzig, Germany)
- Universitäts Klinikum Freiburg (Freiburg, Germany)
- Universitätsklinikum Münster (Munster, Germany)
- Herzzentrum Köln (Cologne, Germany)
- University Hospital Munich (Munich, Germany)
- Friedrich-Alexander University Hospital (Nuremburg, Germany)
With increased understanding of the heart and continuing improvements in prosthetics engineering, computer science, electronics, battery technology, and fuel cells, a practical artificial heart may be a reality in the 21st century.
Heart assist devices

(THORATEC HEARTMATE VENTRICULAR ASSIST DEVICE)
Patients who have some remaining heart function but who can no longer live normally may be candidates for ventricular assist devices which do not replace the heart, but boost its output.

The first heart assist device was FDA approved in 1994, and two more received approval in 1998. While the original assist devices emulated the pulsating heart newer versions, such as the Heartmate II, developed by the Texas Heart Institute of Houston, Texas, provide continuous flow.

These pumps (which may be cetrifugal or axial flow) are smaller and potentially more durable and long-lasting than the current generation of total heart replacement pumps. Several continuous flow ventricular assist devices have been approved for use in the European Union and as at August 2007 were undergoing clinical trials for FDA approval.
VENTRICULAR ASSIST DEVICE:

(Line drawing of how VentrAssist device is implanted )
1.)Natural Heart is not removed.
2.)A short inflow cannula is attached to left ventricle which delivers blood from heart to the device.
3.)The outflow cannula from the pump delivers blood from the device to the ascending aorta, the major artery supporting the body.
4.)Device is then implanted in the "pump-pocket" which is located on the left side of the body, behind the muscles of the abdominal wall and below the rib cage. The drive line from the pump exits from the right side of the abdomen below the ribs.
5.)This connects the pump to the controller and batteries worn on an external belt or backpack.
VentrAssist is the world's leading artificial heart assist device. It is designed as a permanent alternate to heart transplant as well as a bridge to transplant (BTT) or a bridge to recovery (BTR). It is a blood pump that is connected to the left ventricle of a diseased heart to help the ailing heart's pumping action. The device has only one moving part - a hydrodynamically suspended impeller. It is designed to have no wearing parts or cause blood damage. It weighs 298g and is less than 6cm in diameter, making it suitable for both children and adults. The implanted parts of the device are constructed using materials that are fully biocompatible including titanium alloys. It has a diamond-like coating on blood contacting surfaces. The device is powered by an external battery pack with each rechargeable battery set lasting about 8 hours, but also being mains operable. The global market for devices such as VentrAssist will grow to between $7.5 billion and $12 billion annually in the next few years.
MORE PICTURES:
ARTIFICIAL HEART:


ARTIFICIAL HEART PICTURE

ABICOR ARTIFICIAL HEART

ABICOR HEART PUMP

ABICOR HEART

JARVIK-7 ARTIFICIAL HEART

JARVIK 2000 HEART PUMP
HEART ASSIST DEVICES:

VENTRICULAR ASSIST DEVICE PICTURE

ELECTRIC LEFT VENTRICULAR ASSIST DEVICES

PNEUMATIC LEFT VENTRICULAR ASSIST DEVICES

Further Readings For :
1.) HOW ARTIFICIAL HEART WORKS
2.) HEART ASSIST DEVICES
3.) ARTIFICIAL HEARTS



No comments:
Post a Comment