
Confocal microscopy enables the visualization and imaging of fixed as well as living cells and tissues that contain fluorescent probes (antibodies, green fluorescent proteins, dyes, substrates). This technique allows sharply defined optical sections to be collected, from which three dimensional rendering and movies can be created.
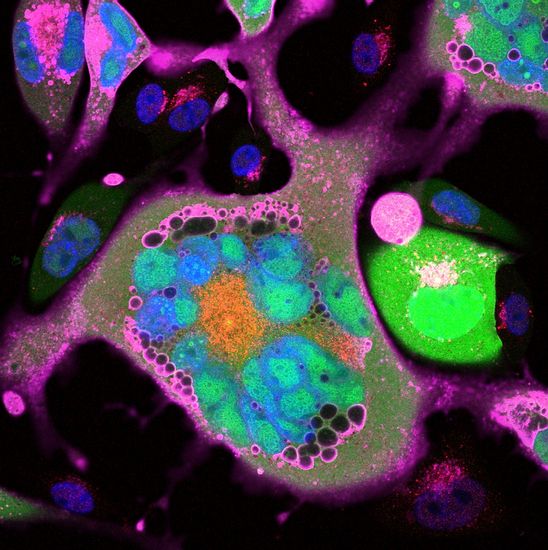
(Human cytomegalovirus infected human endothelial cells. Multicolor Immunofluorescence (IF). Blue: DAPI = cellular DNA. Green = GFP (green fluorescence protein). Red + Magenta = two different viral proteins. Captured with a Zeiss LSM510 laser scanning confocal microscope)
CONCEPT OF CONFOCAL MICROSCOPY
In a conventional fluorescence microscope, the entire specimen is flooded in light from a light source. Due to the conservation of light intensity transportation, all parts of the specimen throughout the optical path will be excited and the fluorescence detected by a photodetector or a camera. In contrast, a confocal microscope uses point illumination and a pinhole in an optically conjugate plane in front of the detector to eliminate out-of-focus information. Only the light within the focal plane can be detected, so the image quality is much better than that of wide-field images. As only one point is illuminated at a time in confocal microscopy, 2D or 3D imaging requires scanning over a regular raster (i.e. a rectangular pattern of parallel scanning lines) in the specimen. The thickness of the focal plane is defined mostly by the square of the numerical aperture of the objective lens, and also by the optical properties of the specimen and the ambient index of refraction.
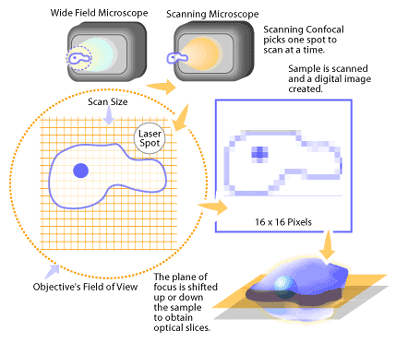
TYPES OF CONFOCAL MICROSCOPES

1.)Confocal laser scanning microscopes

2.)Spinning-disk (Nipkow disk) confocal microscopes




No comments:
Post a Comment